
This page is not the official website. Generated by AI, does not constitute any advice. If needed, please click here to visit the corresponding official website.

Hitec Medical Co., Ltd. is a trusted global supplier of high-quality disposable medical devices for respiratory, anesthesia, urology, infusion, hemodialysis, and PPE applications. ISO 13485 certified and committed to innovation and patient safety.

With over two decades of experience, Hitec Medical Co., Ltd. has established itself as a leading manufacturer of disposable medical devices, serving healthcare providers across the globe.
Since 2003, we've been delivering reliable, innovative medical disposables with a focus on quality and safety.
Our manufacturing processes meet ISO 13485 standards, ensuring compliance with international regulations.
Products distributed in over 80 countries, with participation in major medical exhibitions worldwide.
Hitec Medical offers a comprehensive portfolio of disposable medical devices across multiple specialties, designed for safety, performance, and reliability.
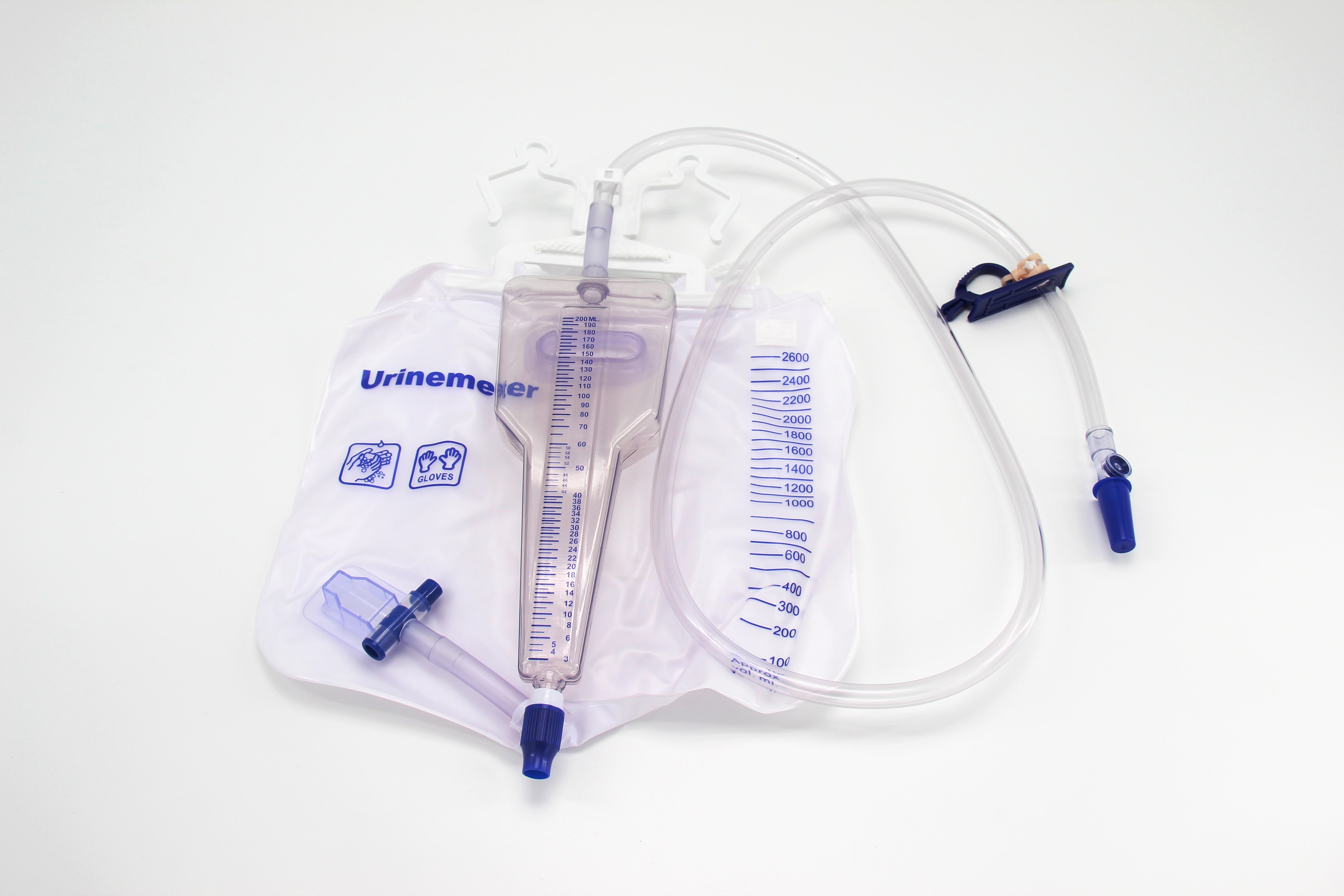
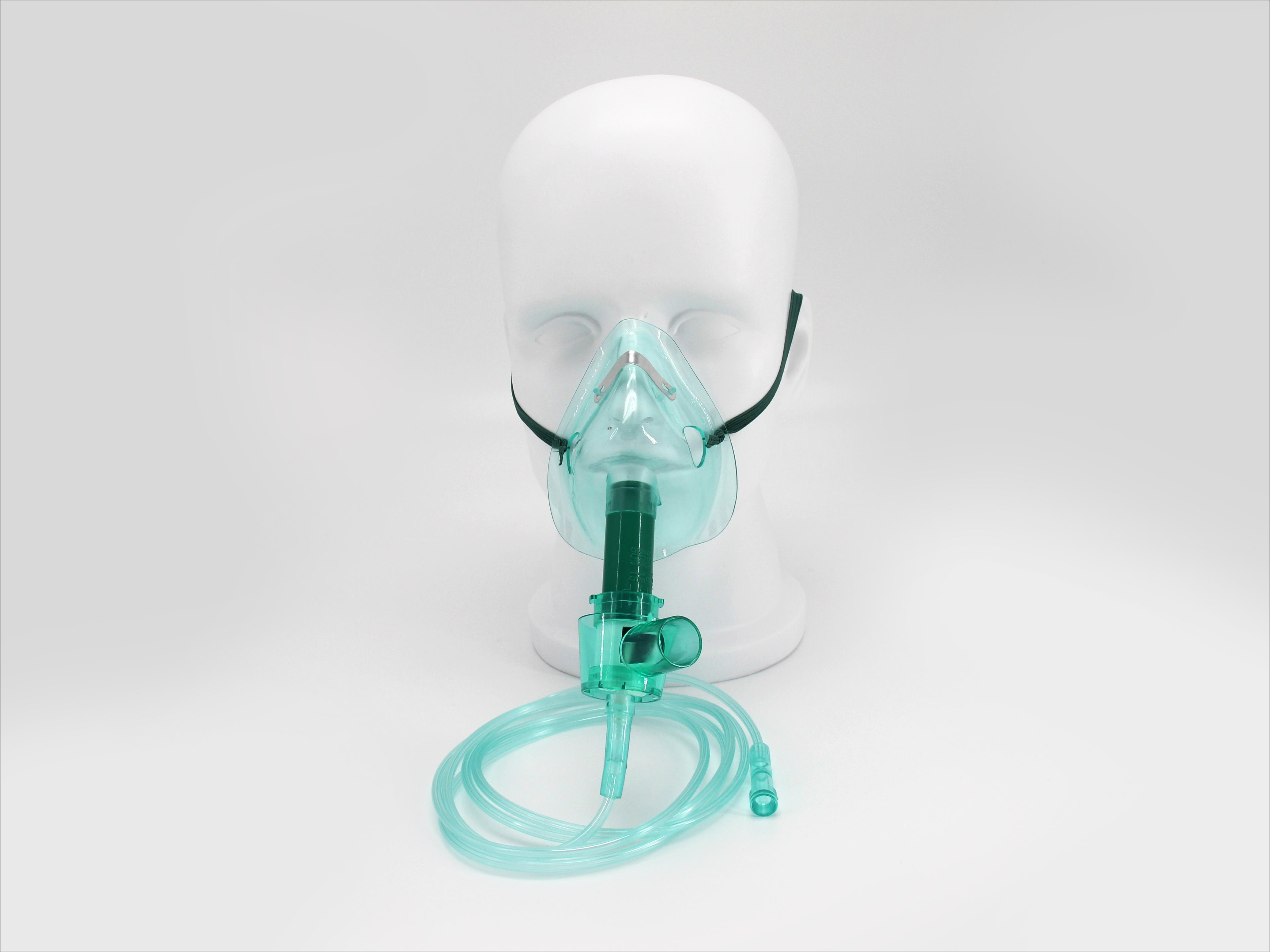





We combine advanced manufacturing, rigorous quality control, and global compliance to deliver medical disposables you can trust.
Our quality management system meets international standards for medical device manufacturing.
Products exported to over 80 countries, with strong partnerships in Europe, Asia, and the Americas.
Continuous investment in research and development to improve product performance and safety.
Flexible OEM/ODM options with customized packaging and branding for distributors.
Assistance with CE, FDA, and other regulatory documentation for market entry.
Regular participation in MEDICA, ZDRAVO, and other leading medical trade shows.
Moscow, Russia | December 8–11, 2025
Booth No. 11E60
Düsseldorf, Germany | November 11–17, 2025
Booth HALL 6 D68-10
Visit us to see our latest innovations in respiratory, anesthesia, urology, and infusion disposables.
Yes, we provide comprehensive OEM and ODM services, including custom packaging, labeling, and product modifications.
Yes, all applicable products are CE marked and comply with EU MDR requirements.
We are ISO 13485 certified and our products meet FDA, CE, and other international regulatory standards.
Please visit the official website to download catalogs or contact the company directly through their online message system.
For inquiries, product information, or partnership opportunities, please visit the official Hitec Medical website.
Visit Official WebsiteNote: Contact forms and direct email/phone details are not publicly listed. Please use the official website for communication.
Notice for Buyers: If you are a buyer, the above information is generated by AI collection and does not constitute any advice. If necessary, please visit the corresponding official website.
For Domain Owners: If you are the domain owner and do not want to be included by fobcompany.info, please contact support@fobcompany.info via your corporate email to cancel. We will cancel your inclusion within 3 business days.
如果您是域名所有者 不想被fobcompany.info收录 请用企业邮箱联系support@fobcompany.info 取消收录 我们将在3个工作日取消您的收录
Service Request: If you are other domain and want to be included, please contact support@fobcompany.info
如果您是其他域名合作 也请联系support@fobcompany.info