Nanotechnology Transforming Drug Delivery
nStrada is a subcutaneous implant that provides long-term, constant release of a wide range of drugs and biologics.
This page is not the official website. Generated by AI, does not constitute any advice. If needed, please click here to visit the corresponding official website.
nStrada is a subcutaneous implant that provides long-term, constant release of a wide range of drugs and biologics.

NanoMedical Systems develops novel drug-delivery devices based on its proprietary nStrada™ nanofluidics platform for sustained release of chronic therapies.

Our nStrada™ devices can be loaded with a variety of drugs including small molecules, peptides, and proteins for subcutaneous implantation. The devices rely on diffusion (passive or controlled) to achieve steady-state drug concentrations within a few days and then maintain constant release for the duration of the implant.
This performance represents a significant improvement over polymer-based implants and depots, which suffer from an initial burst release and require multiple weeks to achieve steady-state concentrations.
Utilizing state-of-the-art semiconductor manufacturing methods and biocompatible materials.
Passive diffusion enables long-term constant delivery without external power sources or mechanical components.
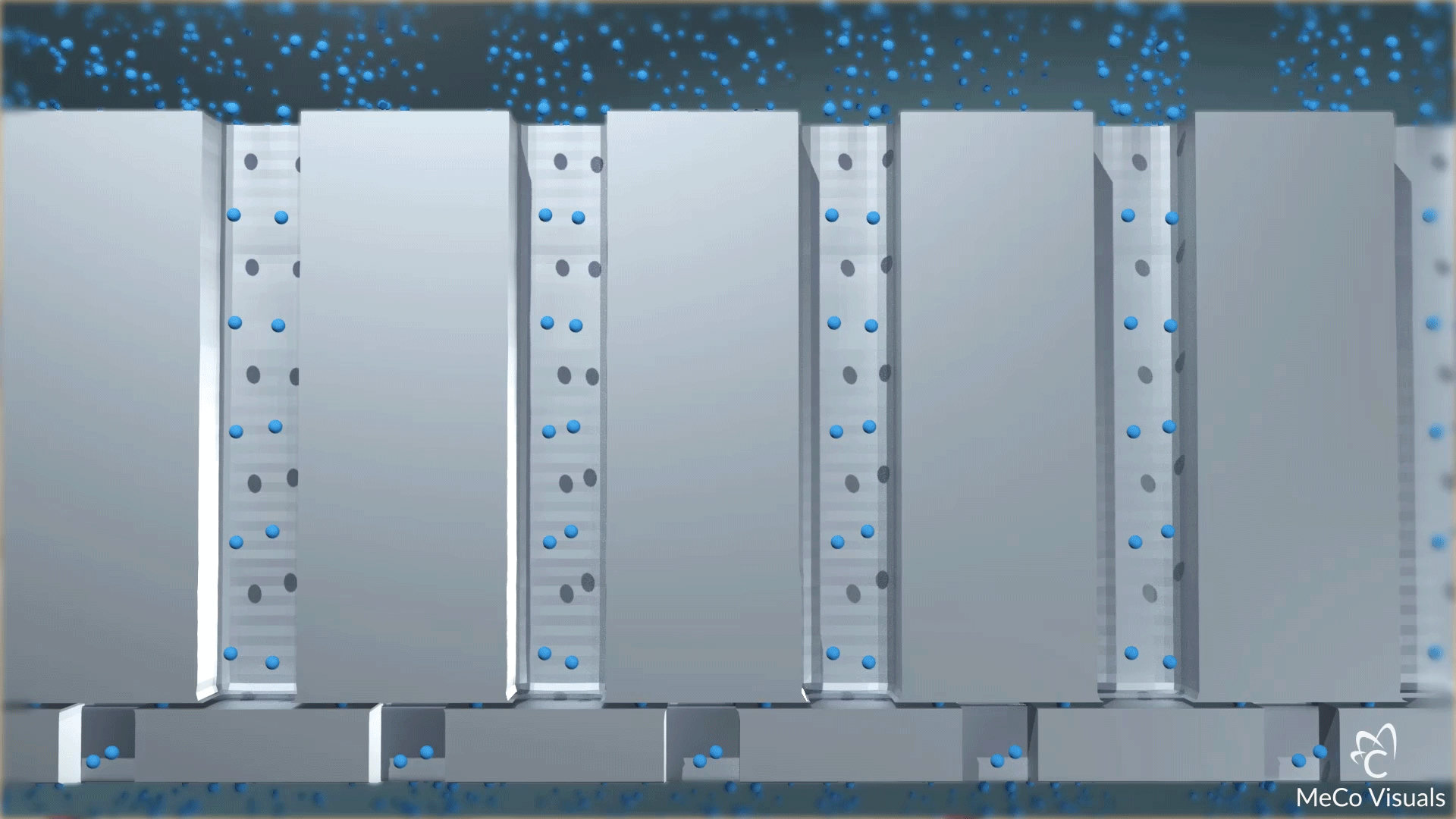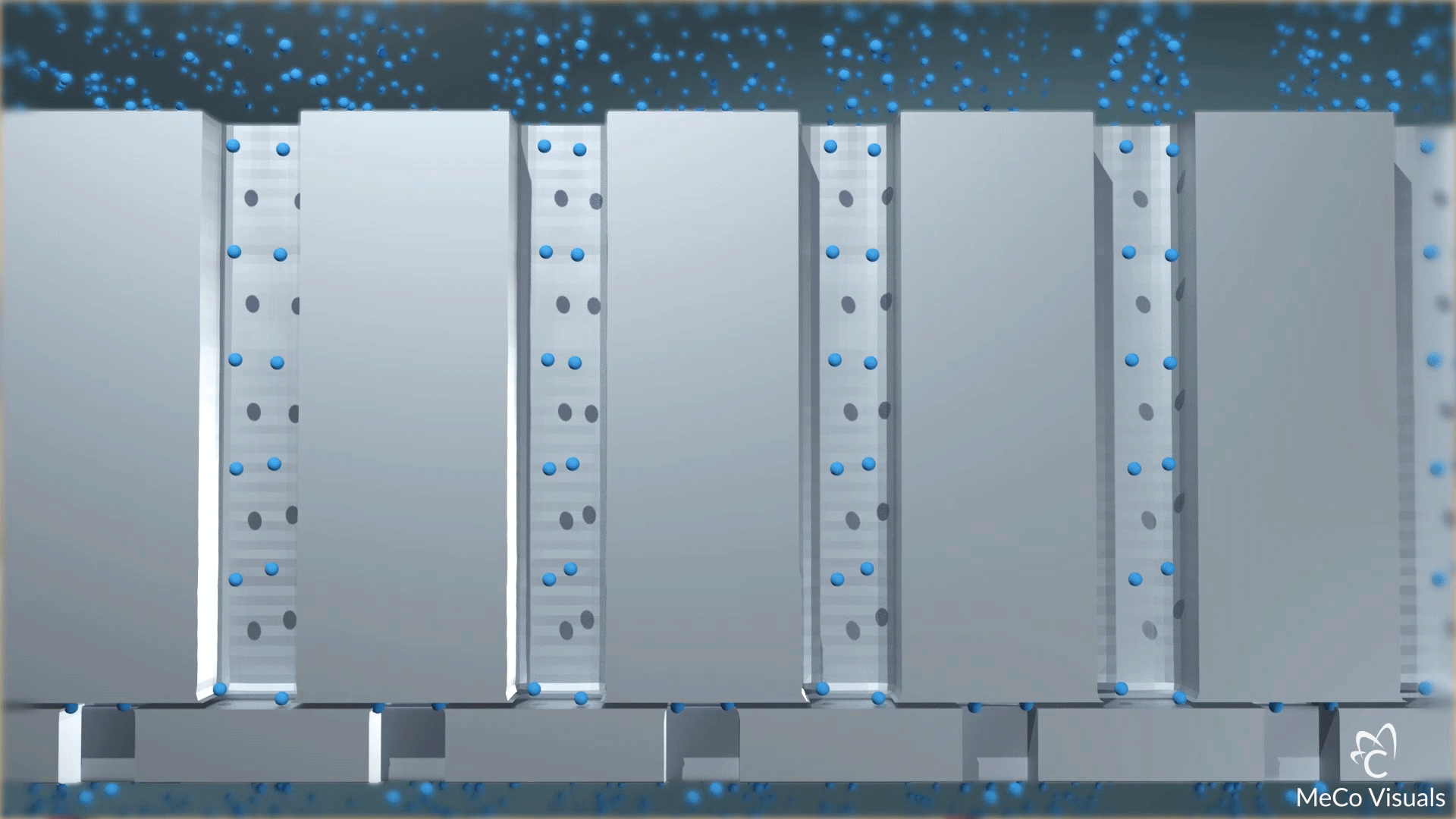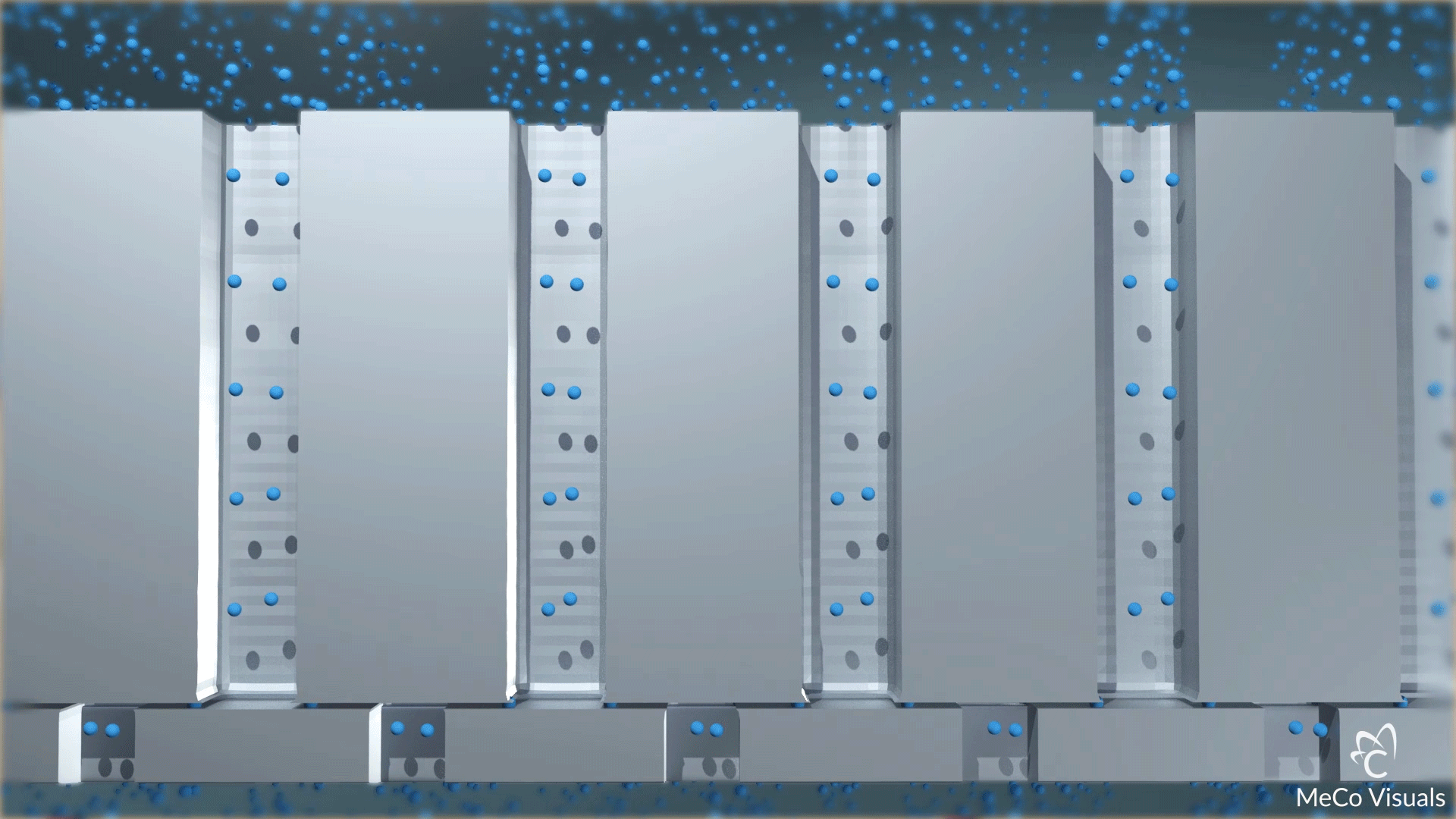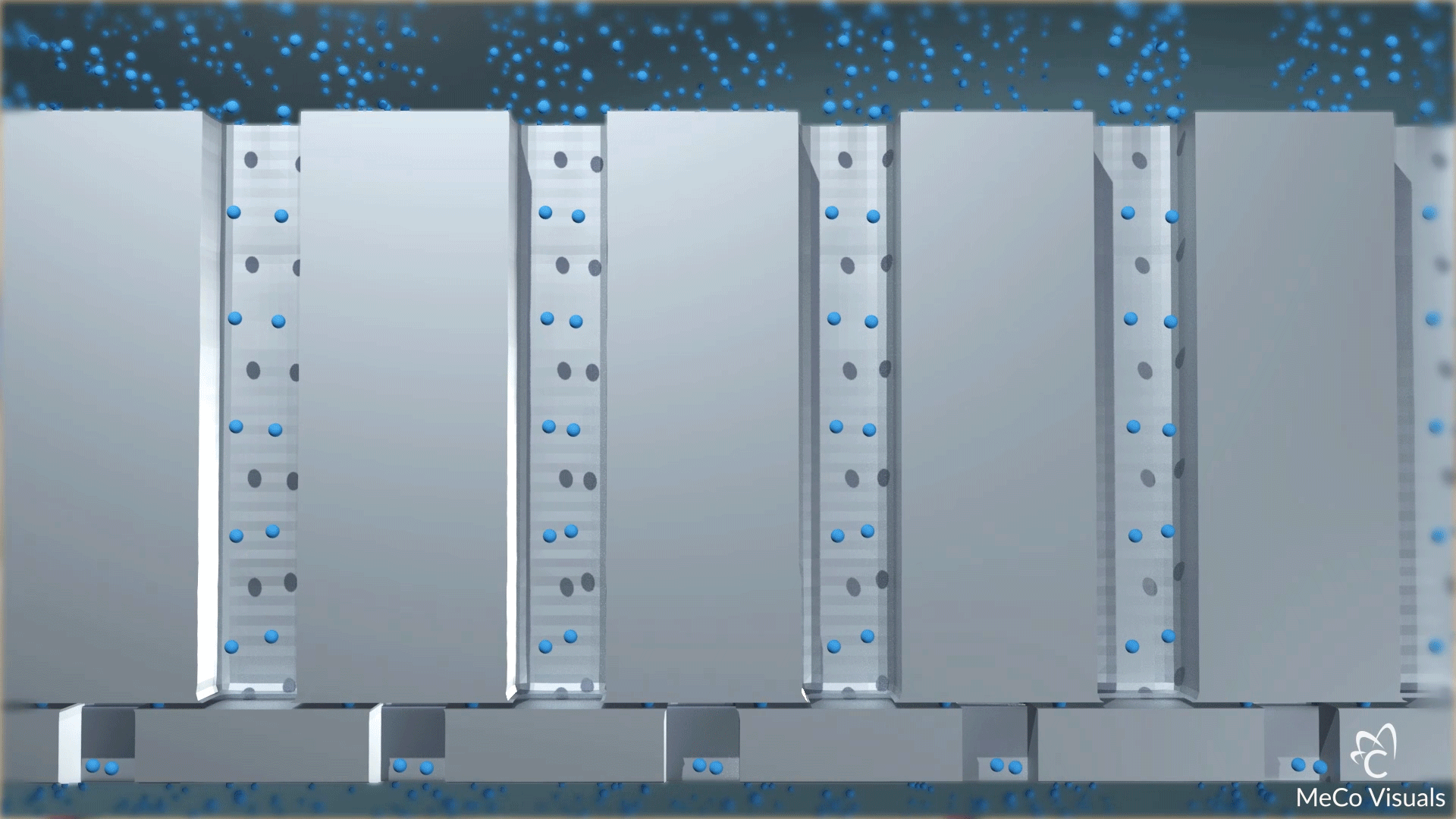
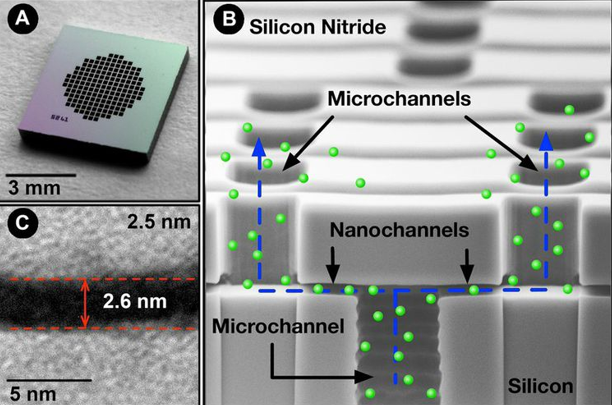
High-density array of atomic-level precise nanochannels regulates drug molecule outflow with unmatched accuracy.
Achieves steady-state concentrations quickly without the initial burst release common in polymer-based systems.
Gen 2 system allows for in-body refilling during routine office procedures, extending treatment duration.
Active control system enables tunable release profiles via remote activation for dynamic therapy management.
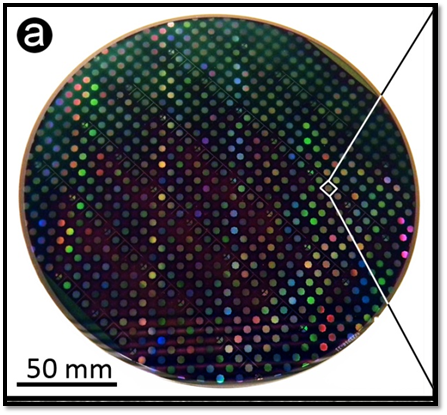
Built using leading-edge chip fabrication processes, enabling high-yield, reproducible production.
Dosing level is set by the number of nanochannels—up to 20 million per cm². Built on leading-edge chip equipment with high-yield processes.
The nStrada™ platform supports a wide range of therapeutic applications through customizable delivery systems.

Passive subcutaneous implant for long-term constant drug release. Ideal for chronic therapies requiring stable pharmacokinetics.

Next-generation system allowing in-body refilling during office visits, reducing implant frequency.
Remotely programmable implant enabling tunable release profiles for dynamic treatment regimens.

Core technology component fabricated using semiconductor processes. Up to 20 million channels/cm².

Platform validated with over 20 drugs (180–149,000 Da). Applicable to small molecules, peptides, and proteins.

Over 10 animal studies across rat, mouse, dog, and monkey models demonstrating consistent performance.

Capsule size and shape tailored independently of dosing parameters for optimal patient fit and comfort.

Regulatory strategy aligned with FDA combination product guidelines for accelerated development.

Future integration with digital health platforms for remote monitoring and treatment optimization.

nStrada devices manufactured in leading-edge chip factories ensuring high reliability and reproducibility.

Collaborate with us to develop novel delivery solutions for your therapeutic compounds.

Proprietary materials and nanostructures provide in-body stability beyond one year.
How nStrada compares to existing drug delivery technologies
| Feature | nStrada | Medici | MicroChips | Polymers/Depots |
|---|---|---|---|---|
| Moving Parts | No | Yes | Yes | No |
| Technology Age | Cutting-edge | 25-year-old | Established | Mature |
| Delivery Type | Continuous | Continuous | Dosed | Burst + Slow |
| Form Factor | Flexible | Single | Single | Variable |
| Refillable In-Vivo | Yes (Gen 2) | No | No | No |
| Burst Effect | No | Minimal | No | Yes |
nStrada is a subcutaneous implant that uses nanofluidics to provide long-term, constant release of drugs and biologics without burst effects.
The device contains a high-density array of precision nanochannels that regulate drug outflow via passive diffusion, tailored to molecule size and dose rate.
The platform supports small molecules, peptides, and proteins (180–149,000 Da), with flexibility for high or low solubility compounds.
Yes, the Gen 2 system is designed to be refillable in vivo during routine office procedures, extending treatment duration.
The system is capable of delivering therapeutics for durations ranging from weeks to over one year, depending on formulation and dose.
nStrada devices are manufactured in leading-edge semiconductor fabs using ISO 9001 processes, ensuring high reliability and scalability.
For more information about our technology, partnerships, or investment opportunities, visit our official website.
Visit Official WebsiteOur Office
NanoMedical Systems, Inc.
HighFlex Technology Center
12708 Riata Vista Circle, Suite A-105
Austin, TX 78727
Notice for Buyers: If you are a buyer, the above information is generated by AI collection and does not constitute any advice. If necessary, please visit the corresponding official website.
For Domain Owners: If you are the domain owner and do not want to be included by fobcompany.info, please contact support@fobcompany.info via your corporate email to cancel. We will cancel your inclusion within 3 business days.
如果您是域名所有者 不想被fobcompany.info收录 请用企业邮箱联系support@fobcompany.info 取消收录 我们将在3个工作日取消您的收录
Service Request: If you are other domain and want to be included, please contact support@fobcompany.info
如果您是其他域名合作 也请联系support@fobcompany.info